Water is essential for all living beings, from the smallest bacteria to the human. It is a perfect medium for all biochemical processes in our body. You probably know that a molecule of water consists of 2 Hydrogen and 1 oxygen atoms. But did you know that it is the only substance that occurs in all 3 states: solid, liquid and gas? And why is it called a universal solvent? Let’s explore some amazing properties of water
Earth, fire, air, and water have long been considered prime elements and essence of all life. Now we know those “natural forces” are not really elements, but something else entirely. Fire, for example, is a chemical reaction between oxygen in the air and fuel such as wood or coal. Earth and air are actually combinations of many different elements.
Water is a molecule, composed of 2 hydrogen atoms and one oxygen atom. But, ancient philosophers got one thing right – water is essential for life. There is a reason why all ancient civilizations were founded next to the rivers. Life, as we know it, depends on water.
Water in the human body
Our body consists of 65% of water. That’s amazing! But what’s more interesting is that certain organs like the brain and the hearth (73%), as well as lungs (83%), depend on water consumption even more.
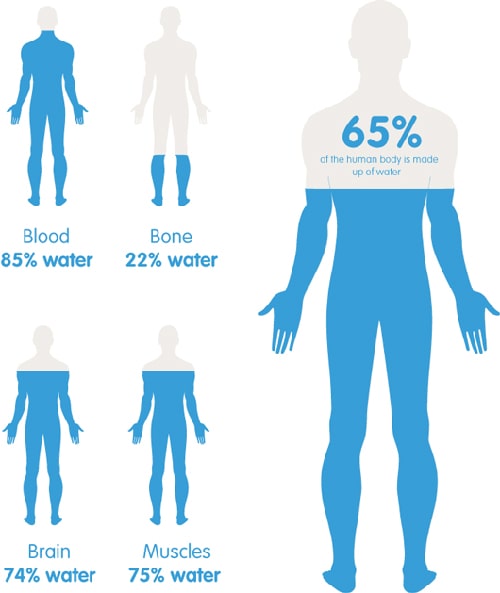
Water is our essential need, we need to drink on average 2L per day just to survive. And in the case of dehydration, our brain shifts to water saving mode. As a consequence, cognitive functions quickly decline to prevent unnecessary “wasting”. In our body, water has multiple roles. Maybe the most obvious is that it regulates our temperature by sweating. But even more importantly, it provides fuel for every cell in our body. It’s also used to metabolize and transport food as well as to flush waste. It forms saliva needed for digestion and lubricates joints. Water is really essential for our everyday functioning.
Chemical structure of water
Structurally, the molecule of water looks like a letter V. The formula for the water (H2O) is probably well known to everyone. Two hydrogen atoms bond with one oxygen atom by what is called a covalent bond. That means that electrons are shared between the oxygen and hydrogen atoms, not transferred.
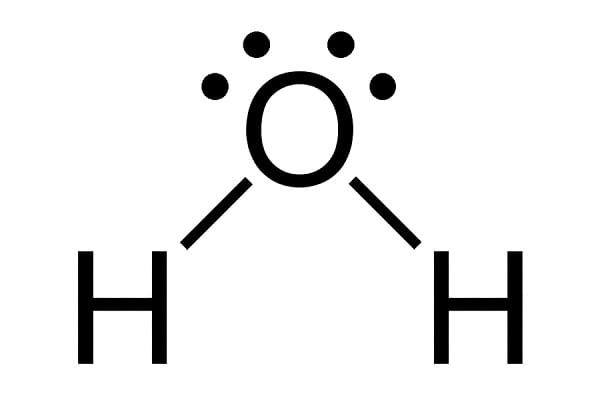
Oxygen is a more electronegative of two, more “greedy” for electrons, so that part of the molecule has a slightly negative charge. Another part (hydrogen part) has a slightly positive charge. This type of the molecule, with differently charged ends, is called a polar molecule. That polarity makes water molecules highly attracted to each other. Positively charged end of one molecule is attracted to negatively charged end of another water molecule. We say that multiple water molecules are connected by hydrogen bonds. That allows for some interesting properties we will discuss next.
Adhesion and cohesion
Cohesion and adhesion define “stickiness” of a substance. Cohesion is an attraction between similar things, while adhesion is an attraction between different things. Water has high cohesion, it is actually highest of all nonmetallic liquids. That means that molecules of water like to stay close together (remember hydrogen bonds?). You can see this property in action if you observe a drop of water on the wax paper. A drop of water consists of many water molecules bonded by a hydrogen bond and it retains its shape.

But there are cases when the attraction to other types of molecules overpowers cohesion.
Did you ever observe water on the glass? It becomes a wet mess, unlike elegant drops we saw before. Why? Well, glass molecules are even more polar than the water, so they become more attractive. This is adhesion. Adhesion and cohesion work together to achieve some really amazing things. One of them is capillary action, movement of water upwards, against the gravity. This is the principle on which the plants transport water around and it also helps with blood circulation in our body.
The universal solvent
A solvent is a thing which can dissolve other things. And water can dissolve more things than any other liquid. That’s why it’s often called the “universal solvent”. Those things which we put in liquids are called solutes.
Since water is polar, it can dissolve other polar molecules and ionic compounds. Examples include table salt, food colors, and sugar. Since they dissolve in the water we call them hydrophilic molecules. Those that do not mix with water, such as oils, we call hydrophobic, water-fearing molecules. They do not dissolve in water since they are nonpolar. Nonpolar molecules interfere with the hydrogen bonding of water molecules. We explored more this topic in our Lava lamp experiment so try it out if you’re interested.
Density and heat capacity
Did you know that water is only substance on the Earth occurring in all 3 forms: solid, liquid and gas? And another peculiarity: The density of the water is higher in a liquid form than in solid (ice). That’s another property unique to the water. But how is that happening? Around 0 degrees Celsius, hydrogen bonds start forming crystalline structures. In crystalline structures, atoms are more spread apart so the density is lower. That’s why the ice floats on the water!

Water has a very high heat capacity. Actually, it has the highest heat capacity of all liquids! That means it can absorb a lot of heat before its temperature rises. The opposite is also true: water cools down slowly. If you’ve ever been around the sea, you know the climate is much more pleasant. Water serves as a buffer that prevents huge differences in temperature.
Since the human body consists of much water, this property also helps us in regulating body temperature. Think of the various temperature conditions we find ourselves in, from freezing winters to scorching summers. We can manage it without many issues because our body uses water as a temperature buffer. If you want to see water’s heat capacity in action, check out 5 amazing balloon experiments where we explored it in details.
And that is all from us but just the beginning for amazing water. If you enjoyed this article, we recommend that you experiment a little. Why don’t you try to make your own plastic? Or go explore osmosis with gummy bears or Diffusion with hot and cold water. Many, many exciting scientific adventures await you.
If you’re searching for some great STEM Activities for Kids and Child development tips, you’re in the right place! Check the Categories below to find the right activity for you.

STEM Science
Videos, guides and explanations about STEM Science in a step-by-step way with materials you probably already have at your home. Find new Science ideas.
Read more
STEM Technology
Videos, guides and explanations about STEM Technology in a step-by-step way with materials you probably already have at your home. Find new Technology ideas.
Read more
STEM Engineering
Videos, guides and explanations about STEM Engineering in a step-by-step way with materials you probably already have at your home. New Engineering ideas!
Read more
STEM Math
Videos, guides and explanations about STEM Math in a step-by-step way with materials you probably already have at your home. Find new Mathematics ideas.
Read more
Psychology
Find out all about development psychology topics that you always wanted to know. Here are articles from child psychology and development psychology overall.
Read more
First year of Child’s Life
Following a Child’s development every month from its birth. Personal experiences and tips on how to cope with challenges that you will face in parenting.
Read more