Today we will combine two fun activities from our childhood: eating gummy bears and learning about osmosis! Just kidding about osmosis being fun, back then it was a hard concept to grasp. But in today’s experiment, we will show you how to learn this important concept in a fun and easy way
Article Contents
1. What is Osmosis?1.1. Solvent, Solute, and Solution
1.2. What is Semi-Permeable Membrane?
1.3. Why Is Osmosis Important?
2. Materials needed for the Gummy Bear Experiment
3. Instructions for making Gummy Bear Osmosis Experiment
3.1. Results of the osmosis experiment
4. What will you develop and learn?
What is Osmosis?
Osmosis is defined as the movement of water molecules from a solution with a higher concentration of water molecules to a solution with a lower concentration of water molecules, through a cell’s semipermeable (partially permeable) membrane. What do we mean by the concentration of water? It’s the proportion of the water in a solution. Let’s talk about that next.
Solvent, Solute, and Solution
Speaking about Osmosis, you will probably often hear about solvent, solute, and solution. So let’s see what they are.
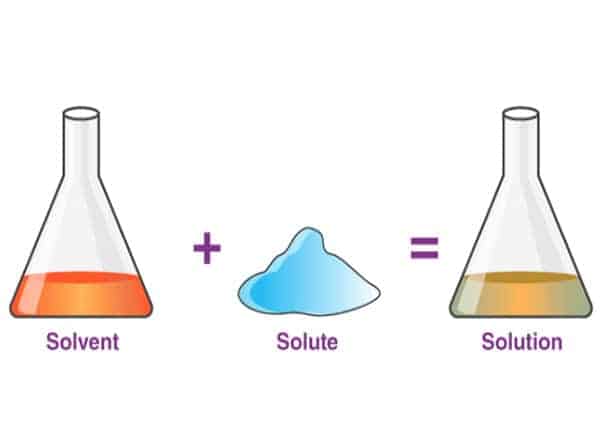
A solvent is any substance that dissolves other substances that we put in it. The most common solvent is water. We know that if we, for example, add sugar to the water, it will dissolve. This is important since, in our organism, water dissolves ions and proteins in our cells.
On the other hand, in our example above, the sugar would be a solute. The solute is a substance dissolved in another substance. So, sugar (solute) dissolves in water (solvent).
And the product we get is called a solution. Solutions can have different concentrations, depending on how much solute we dissolve in a solvent. If we add more sugar to the water, it will be sweeter and denser, more concentrated. However, this solution will now have a lower concentration of water molecules, since there are other things (sugar) in as well.
To summarise – when sugar (solute) dissolves in water (solvent) we get a mixture of water and sugar (solution).
What is Semi-Permeable Membrane?
Think of the membrane as a wall with gaps (it’s semipermeable!). When solutions on both sides of the wall have the same concentration, nothing interesting happens – there is an equal probability water molecules will move from each side of the wall so in the end concentration will stay the same.
However, if we change the balance on one side of the wall, for example, add salt to one side – water molecules will now move from the place where there are more of them (ordinary water) to a place where there are fewer of them (salted water).
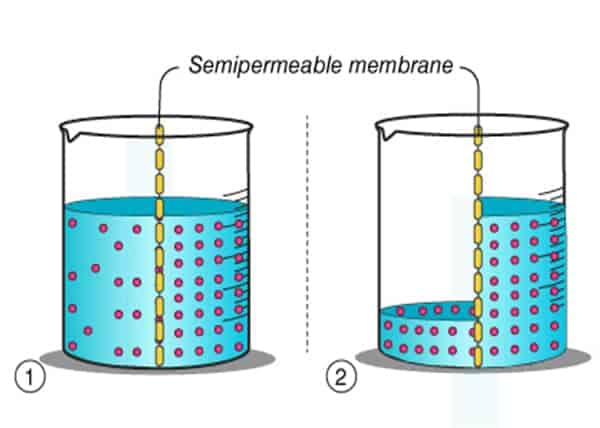
This state of different concentrations is also called osmotic pressure and therefore the amount of liquid will increase on the side with more salt, and decrease on the side where the salt concentration is lower until the osmotic pressure is equalized. The goal is to reach equilibrium, a state where concentrations are the same on both sides.
Here, we have 2 explanations of the process:
- The Mechanical explanation is that molecules of salt are blocking the movement of the water molecules so they are less likely to move from that side.
- The Chemical explanation is that salt molecules consist of ions – Na+ and Cl-. Since water molecules are also partially charged they are attracted to salt molecules and therefore don’t move through the membrane.
Why Is Osmosis Important?
Osmosis is essential for the survival of all living organisms. It allows nutrients and minerals to move inside the cells, through the cell membrane, and also for waste to move out of the cells. For example, plants absorb water from the earth through the process of osmosis.
Try to remember the last time you ate something salty, such as chips. You must have been very thirsty afterward. This is because salt prevents water from passing into the cell through the semipermeable membrane and no matter how much you drink, it is difficult to quench your thirst.
Let’s go now and demonstrate the osmosis process in a simple way using gummy bear candies and different solutions.
Materials needed for the Gummy Bear Experiment

- Gummy bears (gummy candies). You can buy gummy candy in any grocery shop. We have used Haribo gummy bears and they worked well for our experiment. It is not important which gummy candy you use, but we have got reports that some types/brands of gummy candy won’t work well and will just dissolve. Best to have at least 4 gummy bears to make easy comparisons of all experimental results and the original gummy bear.
- Water. 2 deciliters of water will be enough. We will add 1 deciliter to 2 of our glasses.
- Salt. One tablespoon of salt will be enough to act as a solvent and create a concentrated solution.
- Vinegar. We will need 1 deciliter of vinegar to serve us as the second solution and we will add it to the last glass.
- 3 glasses. Since we will have 3 experimental groups, we will need 3 glasses. In the first glass, we will add pure water. In the second glass, we will add water and salt. And in the third glass, we will add vinegar.
Instructions for making Gummy Bear Osmosis Experiment
Check the video at the beginning of the article to see how to conduct this experiment. As mentioned in the required materials section, we used three types of solvent (water, salted water, and vinegar) but you can experiment with any type of solvent.
- Prepare 4 gummy bears (one for every type of solvent, +1 for comparison). Gummy bears are excellent for this experiment because they are made out of sugar, water, and gelatine. Gelatine doesn’t dissolve in water, but it allows water to pass through so it functions as a semipermeable membrane.
- Prepare your solvents. Put pure water in one glass, water with a big spoon of salt into the second glass, and vinegar into the third glass. 1 deciliter of liquid in each glass will be more than enough. You can also experiment with different mixtures, like oil, milk, or soda to see what will happen.
- Put 1 gummy bear into each solution. Leave one gummy bear on the side so you can compare afterward. Leave the gummy bears inside their solutions for a few hours. Check every 3 hours to see the changes.
Results of the osmosis experiment

- After 9 hours, we observed that the gummy bear left in pure water got much bigger than in the other solutions. The water went in! There is just a little bit of water in the gummy bear, so there was big osmotic pressure.
- Gummy bear in salted water got just a little bit bigger. Osmosis at work! Salted water had a lower concentration of water than the pure one, so in this situation, less water went into the gummy bear.
- In vinegar, the gummy bear got bigger, but it also started to fall apart, and that’s because of the acid in vinegar which can dissolve the gelatine.
What kind of solutions did you use and what are the results? Tell us all about your experiment in the comments!
What will you develop and learn?
- Knowledge from chemistry and biology. Osmosis, semipermeable membrane, solutions, etc., all play a big role in the functioning of living organisms. Talking about them will help us in better understanding what is happening on the cell level.
- What is osmosis and how does it work. Without osmosis, there would not be life. So understanding osmosis is important to understand biology.
- Scientific method and conducting experiments. Here, we conducted a scientific experiment with 3 experimental variables (water, salted water, vinegar) and a control variable (gummy bear that we didn’t put into any solution). This enabled us to control every aspect that could influence the outcome of the experiment.
- Learning by doing. We best learn through experience, and here, we conduct our own experiments. So new knowledge while having fun is guaranteed!
We hope you too were enjoying this experiment. If you are in the mood for more great activities, we have some to recommend.
- If you are interested in learning about defusion, a similar process to osmosis, then you can check How to demonstrate diffusion with hot and cold water article.
- We also recommend learning about oxidation and how oxygen reacts with electrons in the Apple oxidation experiment.
- If you are interested in making your own sweet candy, you can learn How to make homemade sugar crystals (Rock Candy).
- And finally, if you are interested in learning about polarity, the chemical property of atoms, you can learn about it in a simple but fun Colorful milk polarity experiment.
Happy experimenting!
If you’re searching for some great STEM Activities for Kids and Child development tips, you’re in the right place! Check the Categories below to find the right activity for you.

STEM Science
Videos, guides and explanations about STEM Science in a step-by-step way with materials you probably already have at your home. Find new Science ideas.
Read more
STEM Technology
Videos, guides and explanations about STEM Technology in a step-by-step way with materials you probably already have at your home. Find new Technology ideas.
Read more
STEM Engineering
Videos, guides and explanations about STEM Engineering in a step-by-step way with materials you probably already have at your home. New Engineering ideas!
Read more
STEM Math
Videos, guides and explanations about STEM Math in a step-by-step way with materials you probably already have at your home. Find new Mathematics ideas.
Read more
Psychology
Find out all about development psychology topics that you always wanted to know. Here are articles from child psychology and development psychology overall.
Read more
First year of Child’s Life
Following a Child’s development every month from its birth. Personal experiences and tips on how to cope with challenges that you will face in parenting.
Read more
5 thoughts on “Gummy Bears Osmosis Experiment”